[ad_1]
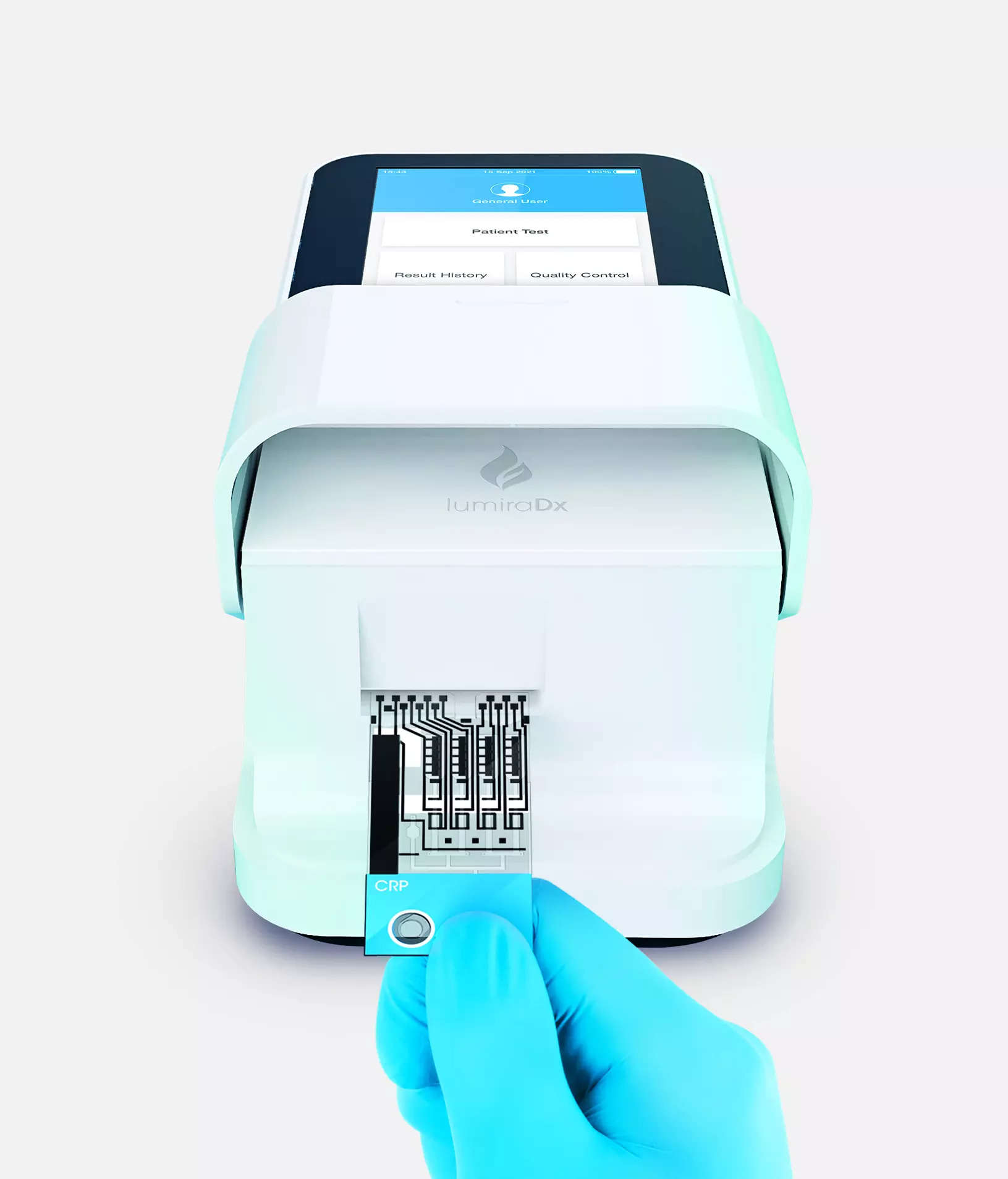
Mumbai: LumiraDx Healthcare Private Limited, a diagnostics testing company, announced it has launched its highly sensitive C-Reactive Protein (CRP) point-of-care antigen test across India. The point of care CRP test can be used in multiple clinical settings to help reduce unnecessary antibiotic prescribing that leads to antimicrobial resistance. From a tiny sample of finger-prick blood, the CRP test gives results within four minutes on the portable LumiraDx platform which weighs just over a kilogram.
In the light of World Antimicrobial Awareness Week and with the new antibiotic pipeline almost dry, reducing antibiotic prescriptions, managing patient compliance to treatment, and reducing self-medication are vital to alleviate the global burden of drug-resistant pathogens. Studies show that point of care CRP testing reduces antibiotic prescribing by 23-36 per cent for respiratory tract infections (RTI)1, and 22 per cent for COPD2. CRP testing can effectively help to reduce unnecessary antibiotic prescribing in primary care.
“The availability of the LumiraDx CRP Point of Care Antigen Test can help to ensure that antibiotics are only given to patients who will benefit from them,” said Yogesh Singh, General Manager, LumiraDx.
Adding to this, Dr Nigel Lindner, Chief Innovation Officer, LumiraDx, said, “Ensuring doctors’ access to crucial actionable CRP test results in care settings is a sustainable way to future-proof our system against antibiotic resistance.”
In India, CRP tests are available in ICUs, paediatric departments, EDs, GPs, OPDs, and other clinical care settings. The Government of India considers AMR as a priority health issue and has undertaken a nationwide initiative by launching an AMR stewardship programme.
[ad_2]
Source link


